Using the Properties Tables
Table A1 gives you gas constants and the properties of the critical-point of different properties
Pure Substance
Definition
Has a fixed chemical composition throughout the system and the process. Phase does not matter.
Equation of State
Note
This relates intensive properties of a pure substance
Can be:
- Algebraic
- Graphical
- Tabular
We can define delta(u) for a pure substance undergoing a quasi-equilibrium process to use it in:
You need to define state using Thermodynamic State
Example
See here for the LE8 example. Beyond the critical point on a T-v diagram, there is the super critical range where gasses start to exist in distinct phases.
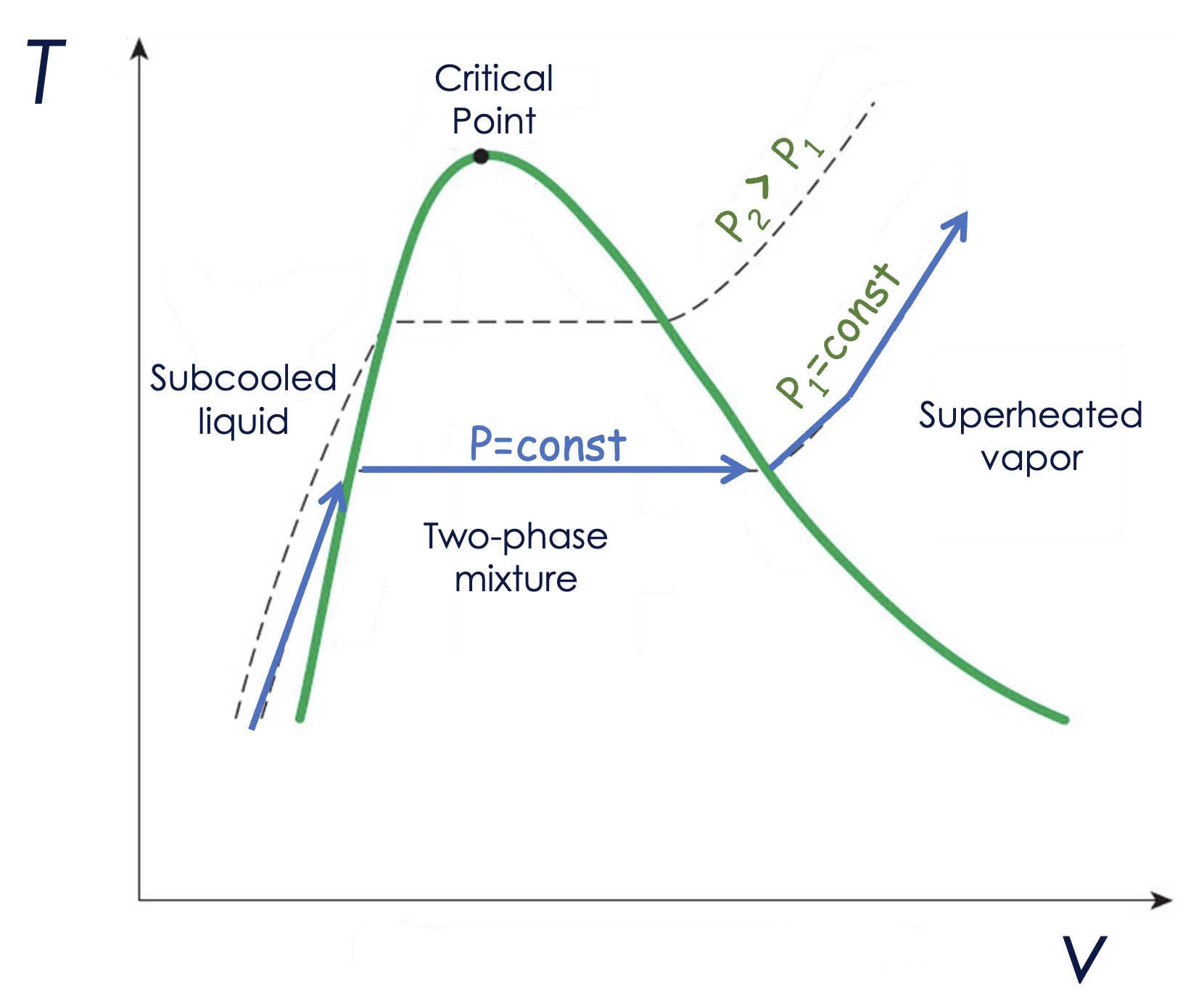
The curve on this diagram is the process path where area under it is the work output.
Sometimes phase changes deal with Enthalpy